PROJECTS
The technology centre is currently cooperating with the Foundation for Biomedical Research at Hospital 12 de Octubre to develop an osteotomy fixation device to improve quality of life for patients and prevent the onset on parastomal hernias.
In Spain, there are approximately 70,000 ostomized patients. These are individuals who, as a consequence of pathologies such as colorectal cancer, have been operated on to connect an external pouch where urine or faeces are collected and arrive via a tube. These are not official registers, although the data was published in Libro Blanco de la Ostomía (1), the White Paper on Ostomy.
An ostomy is a technique that significantly changes the life of a patient subsequent to an operation as it requires specialised care and is associated with consequences such as parastomal hernias with lesions involving the area of the abdomen where an incision has been made. Ostomies, in fact, produce parastomal hernias in 50% of the patients and between 10-15% of them must be operated on to be repaired.
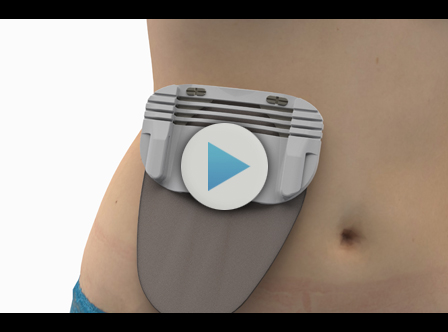
In order to improve quality of life for ostomized patients and prevent these lesions from occurring, Tekniker, member of the Basque Research and Technology Alliance (BRTA), is collaborating with the Foundation for Biomedical Research at Hospital 12 de Octubre in Madrid to design an ostomy fixation device to prevent parastomal hernias.
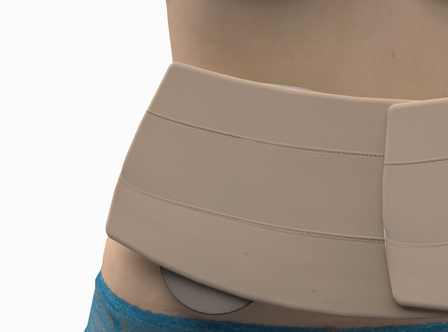
This solution comprises a base that is placed on the abdomen on both sides of the stoma or incision and provides the patient with an easy-to-use and anatomically shaped protective shield. A patent application is currently being filed for the design process. A fixation strip is also provided to adapt the device to the area where the stoma is located.
Within the scope of this project, Tekniker has submitted several proposals to redesign and optimise the product based on its outstanding track record in terms of designing, developing and manufacturing precision equipment.
Moreover, functional and biocompatible materials are being used to manufacture all components that are easy to manufacture according to profitability and product economy parameters with a view to being launched at an industrial at a later stage.
In stage one, the technology centre focused on the functions that the device must perform, on ergonomy, on using different materials and their potential with regard to obtaining scalable parts for industrial production.
Stage two has been more geared towards choosing the most suitable materials, designing the device with all its details and specifying production resources. Tekniker has already assembled the prototypes for the validation process to be carried out with patients by the Foundation for Biomedical Research at Hospital 12 de Octubre.
An industrial feasibility report will be released in the final stages of the project for the purpose of selecting optimum industrial processes and potential suppliers as a function of the number of units to be manufactured in the future.